KIRO® Link
KIRO® Link is a software solution that integrates all Grifols compounding devices with the organization’s EMR or equivalent, manages preparations, connectivity and final label printing.
Overview
For the compounding of sterile preparations of hazardous drugs.
Integration platform or middleware designed to store, transform formats, transfers and display medical data needed for integration and for intravenous preparation workflow management functionalities.
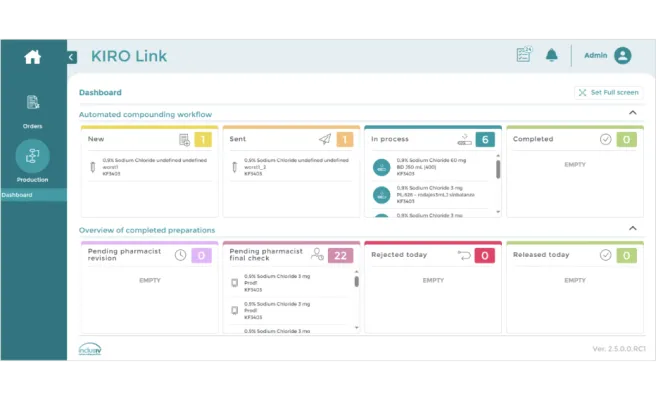
KIRO® Link is a web-based software application that is designed to be accessed from any workstation with connection to the hospital network. KIRO® Link provides access and follow-up information related to the compounding orders (batch and patient specific) sent to Grifols compounding devices (KIRO® Oncology, KIRO® Fill and Gri-fill®).
KIRO® Link’s dashboard shows all the orders sorted by priority and status (pending, in process, completed, to be verified, released, rejected, etc.), providing direct access to the details about each order and/or compounding specifics.
KIRO Link provides access to visualize and print detailed preparation reports for each preparation compounded in Grifols Compounding Devices, users who participated in the process, dosing accuracy details, etc. which can be exported to external software or printed in a printer connected to Kiro Link.
Production reports for specific periods of time or preparation types can be generated in KIRO Link, including key productivity parameters (operation hours, released/rejected preparations, production speed, etc.) and exported as.xml files or preparation outcomes exported to external software.
KIRO® Link can be integrated with standard black and white and color label printers and with label design software to allow only the labels for the batch being compounded in KIRO® Fill to be printed. KIRO® Link will allow these labels to include batch specific information, as well as configurable standard fields.
Compounded preparations are unloaded and manually labeled according to the hospital Standard Operating Procedures, either during the unloading process, after all preparations of a batch have been unloaded, or after a batch has been released from quarantine.
Need help with your sterile compounding plans?
Contact our experts to discuss your sterile compounding needs and future plans. Our team is here to support you.
Want to receive product updates?
Sign up here to stay on top of the latest from inclusiv.