The Solution
A Saint Francis team that included pharmacy leadership and staff began by identifying gaps, researching options for filling them, and planning a new cleanrooms design. Because of the company’s experience in design, engineering, and sterile manufacturing, Grifols was also brought into the process in its early stages.
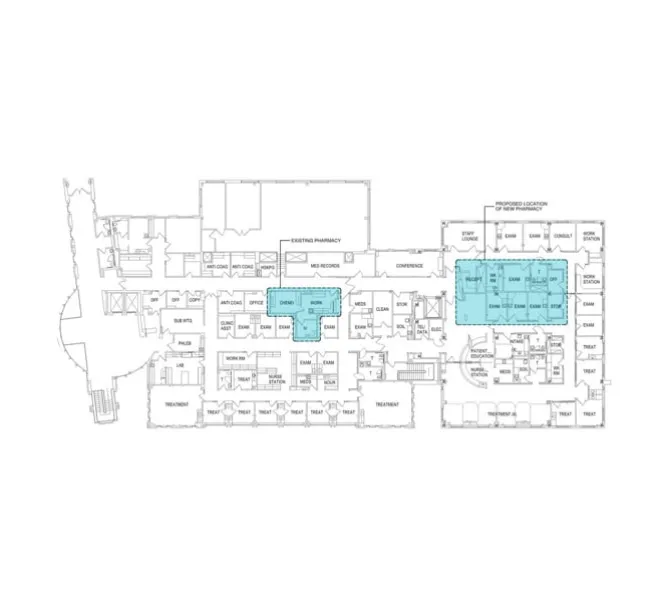
Using knowledge of what is required by USP and the state of Connecticut for compliance, the cross- functional team performed a gap analysis of existing space, compounding activities, and activities to determine changes needed to policies, space, workflow, airflow, and equipment.
Following the gap analysis, Grifols worked in concert with TRO and the internal Saint Francis team to perform a thorough evaluation of the oncology pharmacy workflow of people and product, space, and existing infrastructure. Several layouts were proposed for review and consideration by facilities and clinical decision-makers and influencers.
Once final floor plans and specifications were defined, a request for proposal (RFP) was issued in the May 2015 timeframe.